
Roseline Godbout
Principal Investigator
Godbout Laboratory
Laboratory Overview
The Godbout Laboratory has been doing cancer research for over 30 years, making significant contributions to our understanding of how cellular mechanisms go awry in cancer. Our interdisciplinary approach combines molecular biology, cell biology, and biochemistry to uncover fundamental processes that govern cell fate decisions.
We employ cutting-edge techniques including confocal microscopy, protein-protein interaction studies, and RNA-protein interaction analyses to dissect complex cellular pathways. Our work has particular relevance to aggressive adult cancers like glioblastoma, where understanding the molecular basis of disease can lead to more targeted and effective treatments.
The laboratory fosters a collaborative environment where graduate students, technicians, and research associates work together to advance our understanding of cancer biology. Our research is supported by grants from major funding agencies.
Research Focus Areas
Our laboratory investigates three major research areas that span from fundamental developmental biology to cancer therapeutics, with a focus on understanding how normal cellular processes become dysregulated in cancer.
Overview of Individual Projects
Project 1: DEAD Box 1 (DDX1)
DEAD Box 1 (DDX1) is an RNA helicase that binds and unwinds double-strand nucleic acids (RNA/RNA and RNA/DNA duplexes). DDX1 is essential for embryonic development, with DDX1 knockout in mice resulting in lethality in 2-4 cell stage embryos. In adults, DDX1 protects cells by binding to target RNAs when cells are under genotoxic or environmental stress. Recent work indicates that DDX1 is involved in maintaining the functional integrity of neurons. We are using molecular biology tools combined with cell and animal models to pursue the role of DDX1 in development and disease.
Project 2: FABP7 in Glioblastoma
Glioblastomas are a particularly aggressive type of brain tumour that is resistant to treatment and infiltrates the brain. The median survival time for patients diagnosed with glioblastoma is 14-16 months. We are studying a member of the fatty acid binding protein, FABP7, associated with glioblastoma cell migration, infiltration and poor prognosis. FABP7's preferred ligands are polyunsaturated fatty acids, particularly omega-3 docosahexaenoic acid (DHA) and to a lesser extent omega-6 arachidonic acid. We have recently discovered that FABP7 is highly expressed in long extended microtubes that form connections between glioblastoma cells. Our goals are to investigate FABP7's role and mechanism of action in glioblastoma aggressiveness, and to examine the effects of targeting FABP7 and its fatty acid ligands on glioblastoma growth and infiltration.
Project 3: Prostate Cancer Progression
Prostate cancers have an excellent prognosis until they metastasize. There are no effective methods for treating metastatic prostate cancer. Lipid metabolism has been identified as a key factor in prostate cancer progression. We are using a variety of approaches to investigate how lipid metabolism could be targeted to reduce or inhibit prostate cancer progression.
Key Publications
Recent breakthrough discoveries from the Godbout Laboratory
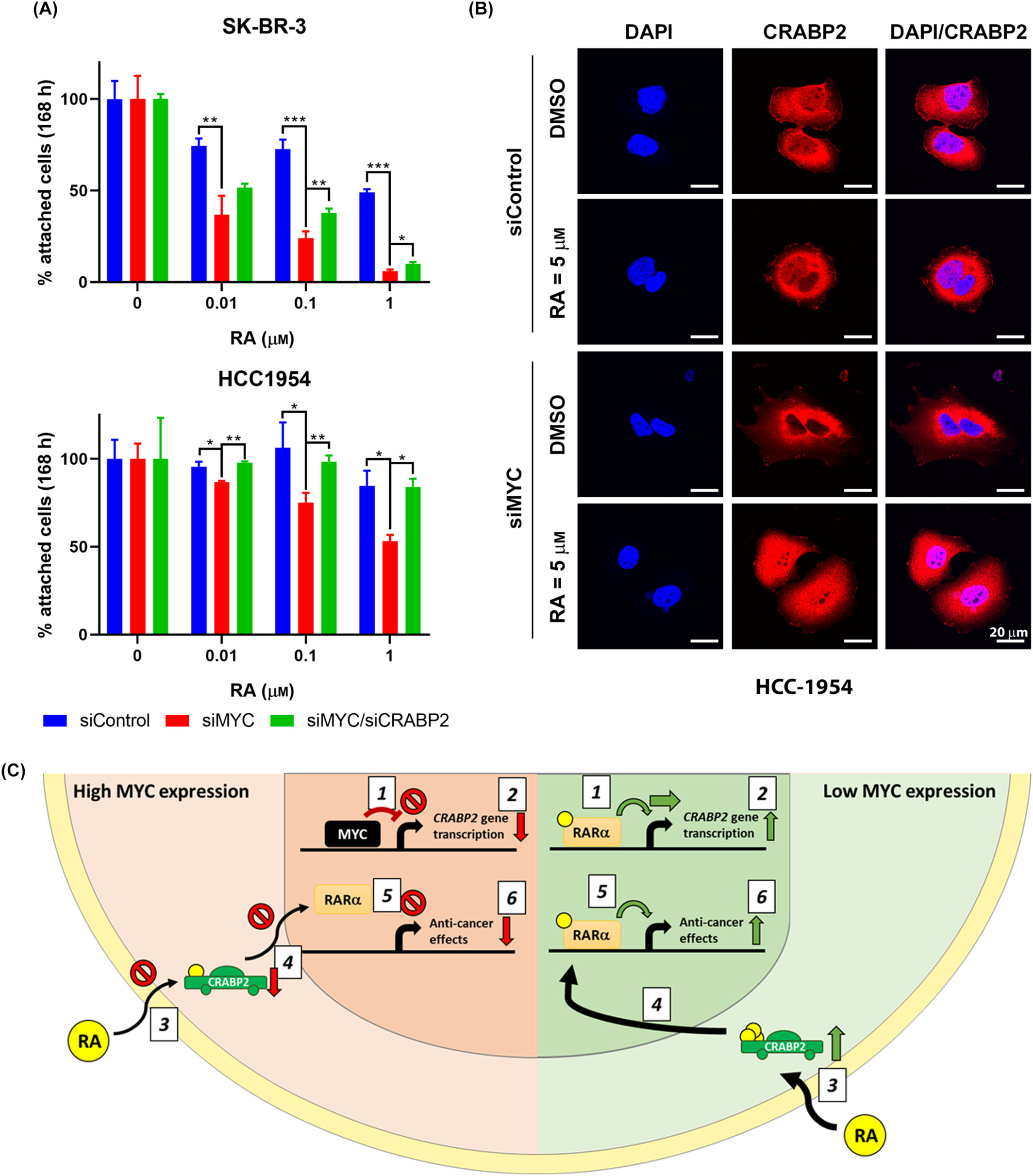
Overcoming retinoic acid resistance in HER2-enriched breast cancers: role of MYC
Significance: HER2-enriched breast cancers are aggressive and often develop resistance to the standard drug trastuzumab. Although these cancers frequently possess the genes necessary to respond to Retinoic Acid (RA) therapy, they often remain resistant to it. In this study, we identified the oncoprotein MYC as a key driver of this resistance. We found that high levels of MYC suppress the production of CRABP2, a transport protein required to shuttle RA into the cell nucleus.
Importantly, we demonstrated that depleting MYC restores sensitivity to RA and, when combined with RA treatment, significantly enhances the effectiveness of trastuzumab. This research suggests that screening for MYC levels could help identify patients who would benefit most from combined RA and trastuzumab therapy.
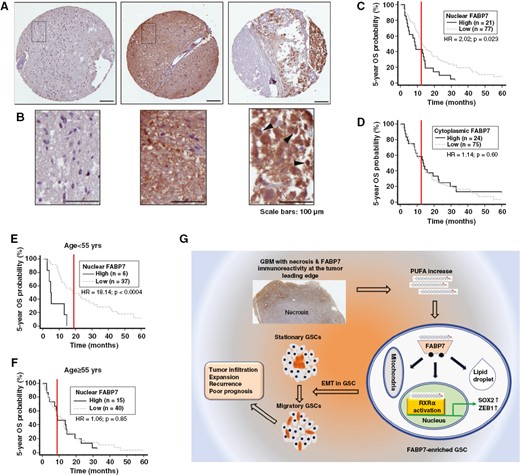
Stationary-to-migratory transition in glioblastoma stem-like cells driven by a fatty acid-binding protein 7-RXRα neurogenic pathway
Significance: Glioblastoma Stem-like Cells (GSCs) are often responsible for tumor recurrence and resistance to therapy. This study identifies a specific subpopulation of "migratory" GSCs that drive tumor infiltration. We discovered that the protein FABP7 is key to this process. When activated by polyunsaturated fatty acids (PUFAs), FABP7 triggers a signaling pathway involving the nuclear receptor RXRα. This pathway upregulates stemness (SOX2) and invasion (ZEB1) factors, transforming stationary stem cells into highly migratory ones.
Inhibiting this FABP7-RXRα pathway successfully reduced tumor infiltration in our models, highlighting a promising new therapeutic target.
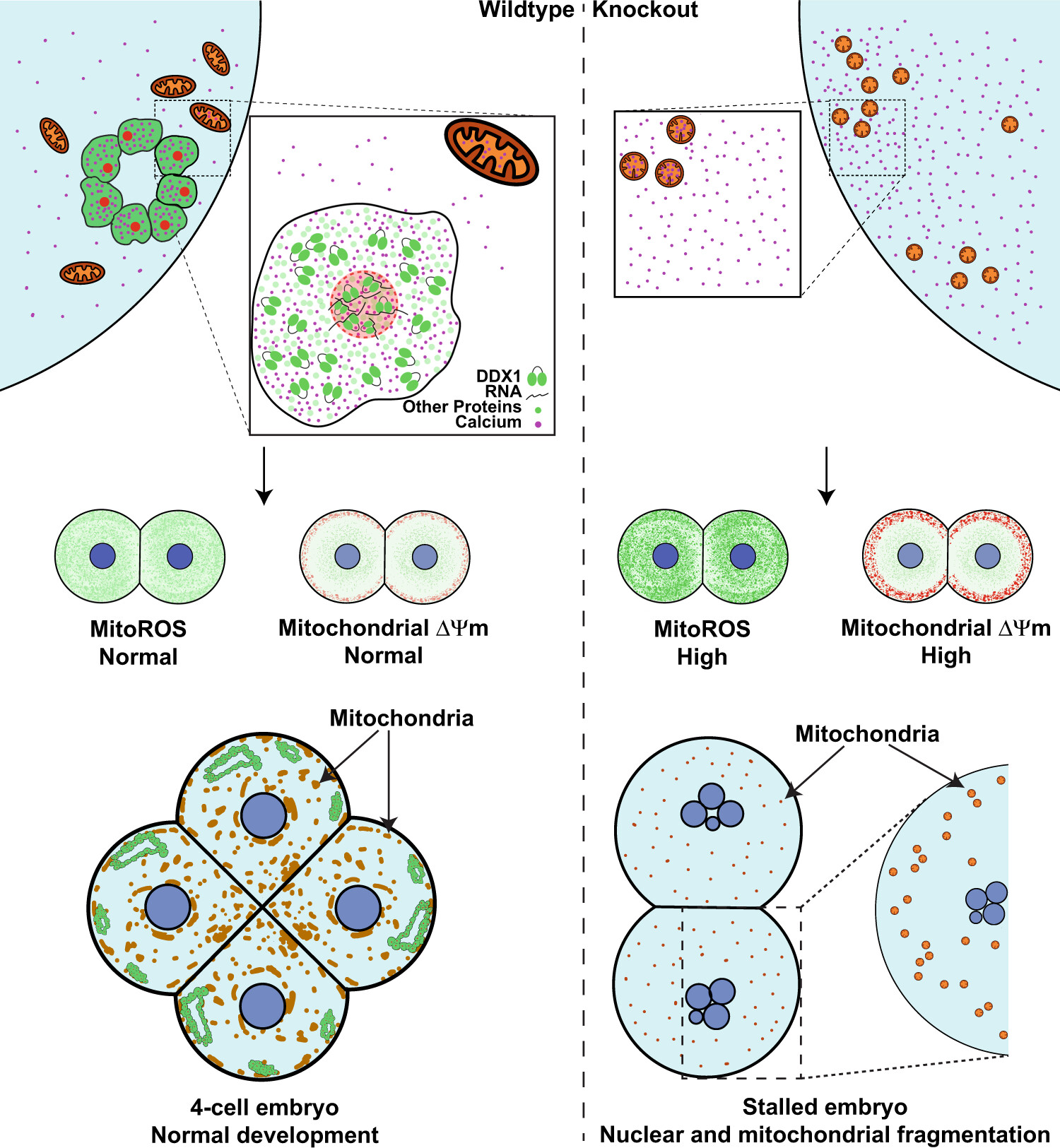
DDX1 vesicles control calcium-dependent mitochondrial activity in mouse embryos
Significance: This landmark study, published in the prestigious journal Nature Communications, reveals why DDX1 is absolutely essential for life. We discovered that DDX1 knockout embryos fail to develop past the 2-4 cell stage due to catastrophic mitochondrial dysfunction.
Using sophisticated live-cell imaging and molecular analyses, we found that DDX1 forms specialized vesicles that regulate calcium-dependent mitochondrial function during the earliest stages of embryonic development. In the absence of DDX1, mitochondria fragment abnormally, produce excessive reactive oxygen species (ROS), and lose their membrane potential - ultimately leading to developmental arrest. This work establishes DDX1 as a critical regulator of the fundamental cellular processes that enable life and provides insights into potential causes of early embryonic lethality and mitochondrial diseases.
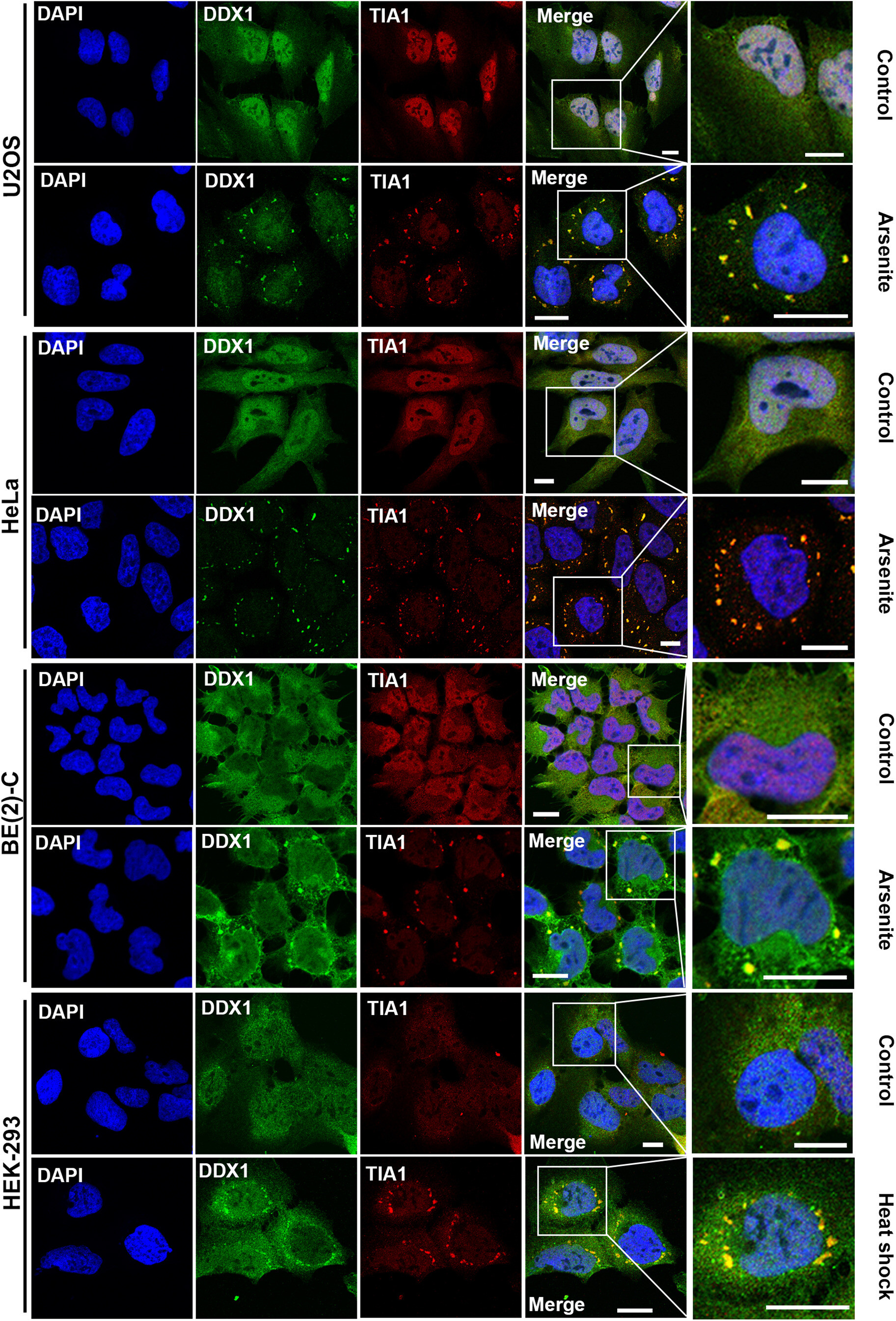
DEAD box 1 (DDX1) protein binds to and protects cytoplasmic stress response mRNAs in cells exposed to oxidative stress
Significance: This groundbreaking study reveals a fundamental cellular survival mechanism. We discovered that DDX1, an RNA helicase, acts as a molecular guardian during cellular stress by forming protective cytoplasmic structures around critical stress response mRNAs.
When cells experience oxidative stress (such as from chemotherapy, radiation, or environmental toxins), DDX1 rapidly relocates from the nucleus to the cytoplasm where it assembles into distinct vesicles containing stress-responsive mRNAs. These DDX1-containing vesicles protect essential transcripts from degradation and ensure their translation into proteins needed for cell survival. This work explains how cells maintain protein synthesis during stress conditions and has important implications for understanding chemotherapy resistance in cancer, neurodegenerative diseases where oxidative stress is prevalent, and normal aging processes.
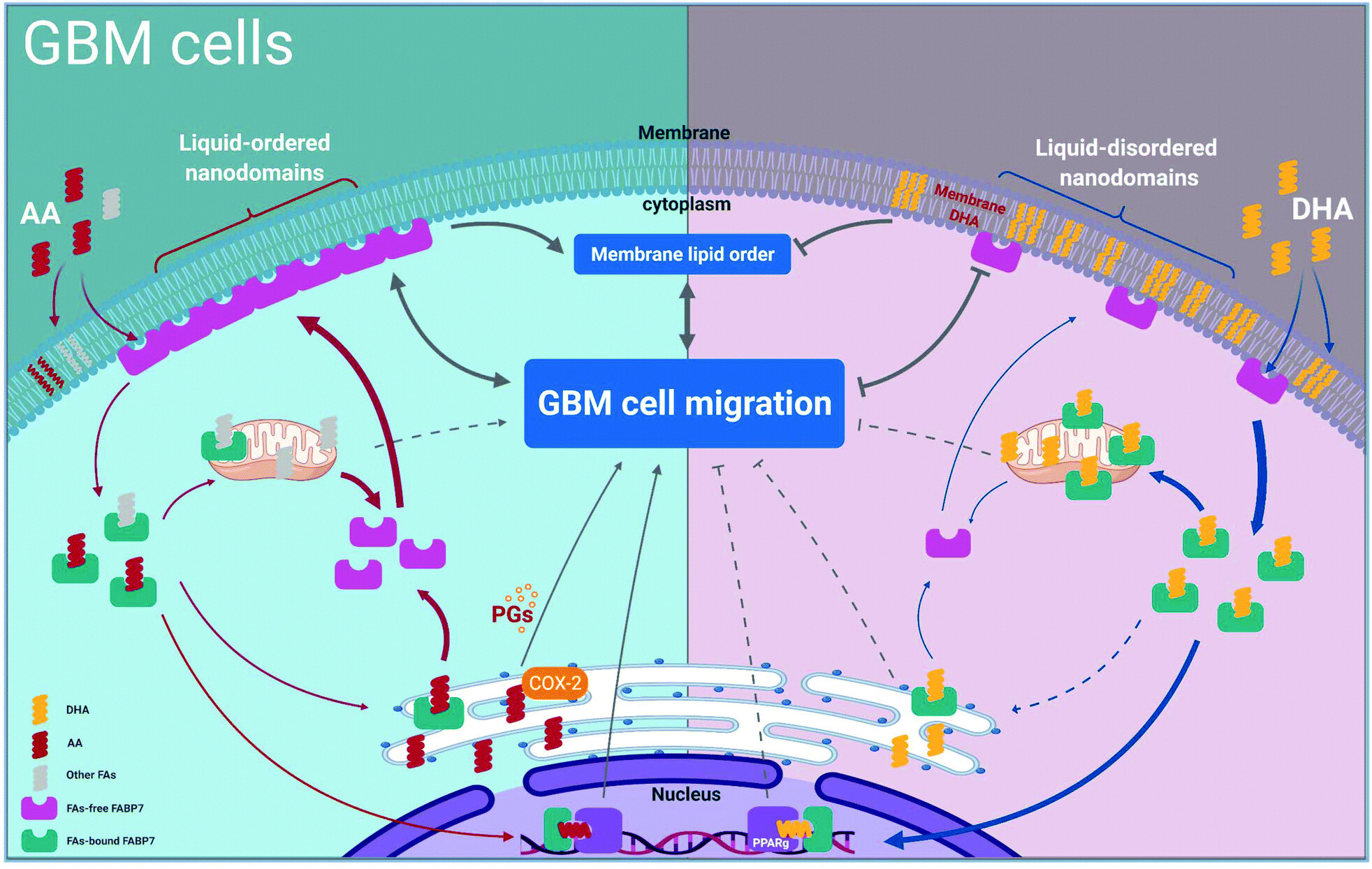
Super resolution microscopy reveals DHA-dependent alterations in glioblastoma membrane remodelling and cell migration
Significance: This study provides critical insights into how docosahexaenoic acid (DHA), an omega-3 fatty acid, affects glioblastoma cell behavior at the nanoscale level. Using super-resolution microscopy, we discovered that DHA induces dramatic changes in membrane organization, creating distinct liquid-ordered and liquid-disordered nanodomains.
These findings reveal a novel mechanism by which dietary fatty acids can influence cancer cell migration and invasion. The work demonstrates that DHA treatment reduces the formation of tunneling nanotubes (TNTs) - specialized membrane structures that glioblastoma cells use for intercellular communication and migration. This research opens new avenues for understanding how fatty acid supplementation might be used as an adjuvant therapy to reduce glioblastoma's aggressive infiltrative properties.
Research Techniques & Methods
Molecular Biology
- • Western blot analysis
- • Co-immunoprecipitation
- • RNA-protein interactions
- • Yeast two-hybrid analysis
Cell Biology
- • Confocal microscopy
- • Live cell imaging
- • Flow cytometry (FACS)
- • Cell cycle analysis
Specialized Techniques
- • Nuclear body analysis
- • DNA damage assays
- • Tissue culture methods
- • Biochemical purification